60 Which of the Following Has a Zero Dipole Moment
Select the molecule among the following that has a dipole moment. 60 C 180 D 109.

Which Of The Following Set Of Molecules Will Have Zero Dipole Moment
Zero die paul moment.
. 80 while in case of itself and its great or H and H tool tractor. So you know in this case the boy moment that a positive direction and our cancel with each other. Be easier to as zero die paul.
A HCN B NH3 C SO2 D NO2 E PF5. Its zero dipole moment heads. This is because this cultural structure of trans two Baldinis moving is see you will want to see parents.
Cheatsheets Important Diagrams Mindmap Common Misconceptions Problem solving tips. As the 3 bonds are in a single plane dipole moments cancel each other with net dipole moment equal to zero. HCN 1 See answer Advertisement Advertisement.
The value of Au for the combustion of one mole of toluene at 300 K is. A NH3 B NO2 C PF5 D SO2 E HCN. Thats the age three age and ch three.
Which of the following molecules have a nonzero dipole momentthat is are polar molecules. The question is this molecule has zero dipole moment. Which of the following has a zero dipole momentA.
12Enthalpy change of combustion of toluene C7H3 at 300 K is -3910 kJmol. One is Clf second is PCL three. The two bond dipole moments CO are equal and opposite in direction.
Cis isomer has more dipole moment than trans isomer because it has two similar groups on same side of double bond. We know that a polar bond has two poles positive and negative pull like a magnet and I pulled moment of a molecule can be calculated only when its structures and. So youll have one going this way.
Sum of bond dipole moments will give the net dipole moment for a molecule. Some of the ipod moment is not zero. The relatively high boiling point of HF can be correctly explained by which of the following.
ClO2 - bent shape. Todd is silicon tetrachloride and fourth is saved seal three. View chapter Shortcuts Tips.
Dipole moment of ch4 is zero the ch4 is tetrahedral in shape thus each bond pair are at equal distance that is they are symmetrically arranged hence each dipole moment of bond balance. A SO b HCN c PF d NH a SO b HCN c PF d NH Previous question Next question. Therefore option A and C are the correct answers.
Okay so we are going to discuss this molecule have zero tribal woman type of moment molecules which have that is some military molecules and also have same surrounding attempts Like us in that BF three CH two cl 2 Will not have zero terrible moment. D HF is much less soluble in water. This problem has been solved.
PF5 has a zero dipole moment as it doesnt have a lone pair of electrons. Which of the following molecules has a zero dipole moment. Therefore they cancel each other resulting in zero net dipole moment.
Since uh chlorine is election I get it. Therefore all these molecule has die paul moment. 560 liters E 374 liters.
C HF molecules have a smaller dipole moment. When a solution of potassium dichromate is added to an acidified solution of ironII sulfate the products of the reaction are. Which of the following has zero dipole moment.
We have to hide regions. Which of the following has a zero dipole momentA. And we have given four different molecules.
View solution Which of the following has zero dipole. In this problem we have to find the molecule which has zero dipole moment. In the trans isomer the terminal groups are on the opposite sides of the double bond.
These rechargeable batteries are used in consumer electronic goods. This is because the individual dipole moments cancel out because of the symmetrical tetrahedral shape of the molecule. 0 -7810 kJ 0 -3905 kJ 0 -1950 kJ 0 -975 kJpls answer it as fast as possible pls.
Chemistry questions and answers. Which of the following has zero dipole moment. Which of the following has a zero dipole moment.
So this Parsi is the one that has a dipole moment of zero and were partied. Carbon tetrachloride and tin tetrachloride have tetrahedral structure and zero dipole moment because all the bond dipoles cancel each other which gives net dipole moment zero. CCl4 is a symmetric molecule and hence even if each bond has a dipole moment the algebraic sum of the dipole moments of all the four bonds cancel out resulting in zero dipole moment.
Solutions for Chapter 24 Problem 60E. Which of the following molecules has no dipole moment. Carbon tetrachloride molecule has zero dipole moment even though C and Cl have different electronegativities and each of the C - Cl bond is polar and has some dipole moment.
And one in this general direction Sochis will cancel each other out and the sum is equal to zero. BCL two has zero dipole moment because it has linear ship and the vector sum of type all moment. So answer for this problem is option is correct.
In tetra-atomic boron trihydride BH 3 the dipole moment is zero but that of ammonia NH 3 is 149D. A HF gas is more ideal. CO2 has zero dipole moment due to its linear shape.
A CO2 B NH3 C H20 D all E none. The liquefied hydrogen halides have the normal boiling points given above. B HF is the strongest acid.
Chemical Bonding and Molecular Structure. And the electrons are shared evenly throughout the whole molecule as a whole. This is because BH 3 has a symmetrical structure and the 3 B-H bonds are placed at an angle of 120 degrees to each other.
View solution View more. The axial P-F bonds cancel each other. Trans has no or zero dipole moment than cis isomer.
Which of the following has a zero dipole moment. The geometry is trigonal bipyramidal. So the dipole gets added thus cis isomer is more polar than trans.
If over heated or over charged they. A CO2 B SeO3 C XeF4 D SF4 E BeCl2.

Which Of The Following Has Zero Dipole Moment

Question 58 1 18 57 The Molecule Which Has Zero Dipole Moment Is 1 Ch3cl 2 Nf3 4 C102 Chemistry Equilibrium 11264867 Meritnation Com
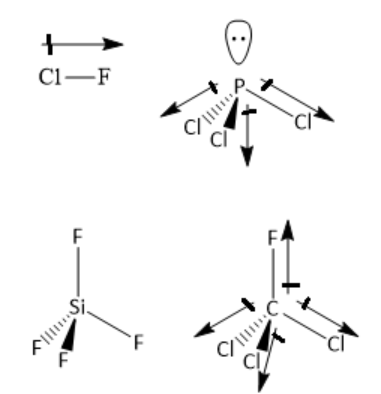
Which Of The Following Has Zero Dipole Moment A Clf Class 11 Chemistry Cbse

Comments
Post a Comment